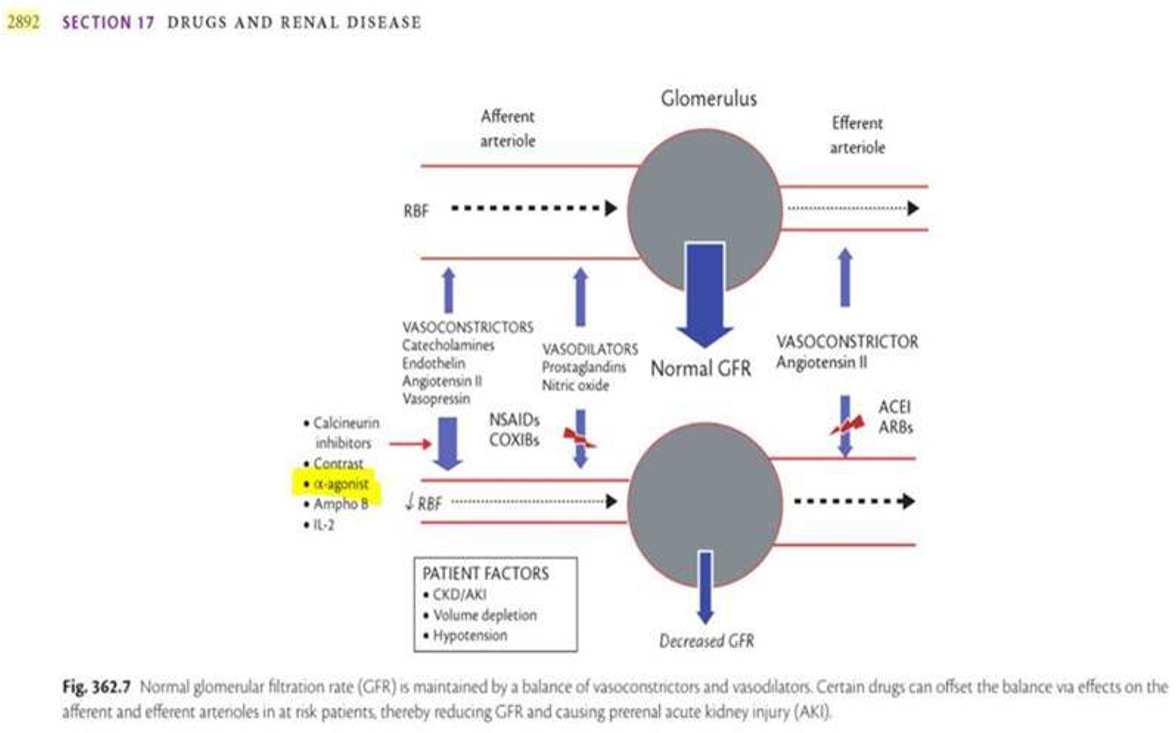
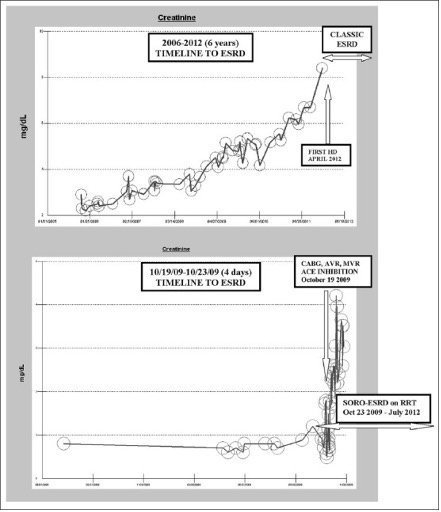
Study 1: Demonstrates That Midodrine Can Rapidly Induce Severe Kidney Injury.
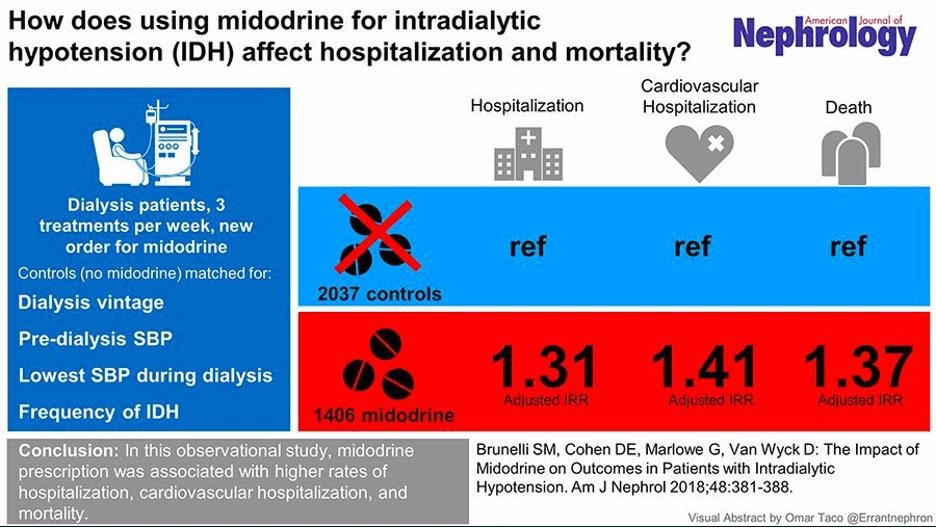
A randomized controlled trial, the gold standard for proving causation, provided compelling evidence that midodrine can cause severe kidney injury/failure. Patients who received midodrine in this study experienced a dramatic rise in creatinine levels, increasing from 0.99 to 3.02, a clear indicator of severe kidney injury . In contrast, the control group showed no such changes. Alarmingly, seven patients in the midodrine group died during the study, while no deaths occurred in the control group.
This study stands as one of the strongest pieces of evidence linking midodrine to kidney failure. The patients in the study were carefully matched across numerous factors, significantly reducing the likelihood that these findings were influenced by other variables.

Note: This image was created to summarize the study's results. Although it was not part of the original publication, the data presented in the figure is directly derived from the study's findings.
Source